In the midst of the COVID-19 vaccines rollout, clinicians and researchers are working to assess next generation COVID-19 vaccine candidates, and as part of that effort, researchers are presenting alternatives to measure a vaccine’s effectiveness by using immune markers that a person develops in their blood after inoculation. Dan Barouch, MD, PhD (Center for Virology and Vaccine Research, BIDMC) spoke to how trials so far suggest that levels of an antibody called immunoglobulin G (IgG) could serve as that proxy indicator.
Nature – January 8, 2021
Search for better COVID vaccines confounded by existing rollouts
Global health researchers breathed a collective sigh of relief last month after nations across Europe, North America and elsewhere issued emergency approvals of the first COVID-19 vaccines. But as the shots are rolled out, clinicians are scrambling to work out how to evaluate dozens of other, earlier-stage vaccine candidates. These could be less expensive, have fewer side effects or be easier to administer than those now in use — and they would bolster the world’s supply of COVID-19 immunizations, ensuring more rapid distribution to all countries.
The trouble is that finding would-be participants for placebo-controlled clinical trials has become more of a challenge. In these trials, half the volunteers receive a dummy shot and half the real thing, but neither participants nor researchers know who received which one until after the trial. People are less likely to chance receiving a placebo when they could get one of the various vaccines now authorized, two of which prevent COVID-19 with about 95% efficacy.
As it is, many people taking part in placebo-controlled trials have already asked to drop out to ensure that they get immunized.
“The landscape is changing,” says Scott Halperin, director of the Canadian Centre for Vaccinology at Dalhousie University in Halifax, who is leading trials of two COVID-19 vaccines in human testing. “Once you have a vaccine that is available,” he notes, “a placebo-controlled trial is no longer ethical or acceptable.”
Analysts expect first-generation vaccines to be widely available in the next 6–12 months in most high-income countries and in some parts of the developing world. Next-gen vaccine makers are therefore considering ways of proving their products’ effectiveness without placebo comparisons. “The window is closing,” says immunologist Robin Shattock of Imperial College London, whose own COVID-19 vaccine is in its first phase of human testing at four sites across southern England.
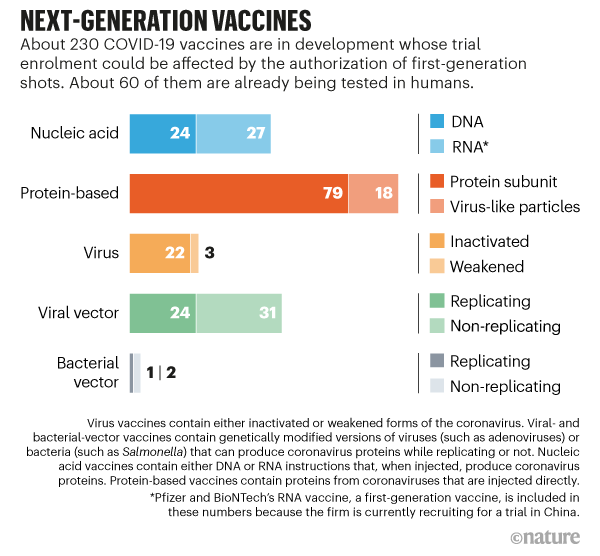
At present, around 60 follow-on vaccine candidates are in human testing, and another 170-plus are in various stages of preclinical evaluation (see ‘Next-generation vaccines’). Some are built on genetic technologies and, when injected, help to produce coronavirus proteins inside the body that trigger immunity. Others contain coronavirus proteins, inactivated forms of the virus or other types of viruses modified to carry genetic instructions for making coronavirus proteins. “On a global level,” says Shattock, “the ones that will win out long-term are the ones that are going to be most stable and cost-effective.”
Recruitment challenges
Already, the mass rollout of vaccines to certain populations is beginning to put the squeeze on trial recruitment. For example, Novavax, a vaccine company in Gaithersburg, Maryland, is trialling a vaccine that uses purified virus proteins, an established approach that offers potential safety advantages. Such vaccines can also be stored in refrigerators, allowing for distribution using standard vaccine supply-chain channels. But in a 30,000-person trial launched by Novavax late last month, health-care professionals — a major target of earlier vaccine efficacy studies because of their elevated infection risk — are no longer highly sought recruits, according to Gregory Glenn, the company’s research and development head. That’s because many are receiving first-generation shots.
And older individuals or people with underlying medical conditions — two groups in particular need of vaccines because of their susceptibility to severe complications from COVID-19 — could become increasingly difficult to sign up. That will especially be the case if governments follow the lead of US states such as Florida and Texas and make those groups eligible for inoculation. Taking these groups out of the pool of potential trial participants “inevitably will make recruitment more challenging”, says C. Mary Healy, an infectious-diseases specialist at Baylor College of Medicine in Houston, Texas, who is involved in the Novavax study.
Trial designers have devised a few workarounds to incentivize participation. In some placebo-controlled studies, for instance, two people receive active vaccines for each one who gets a dummy shot, instead of the usual even split. The approach allows companies to gather more safety information about trial products. That’s because more participants receive an active dose and so can experience adverse reactions. As an added bonus, prospective study subjects are more willing “to roll the dice if it’s a two-out-of-three chance” that they will receive a real shot, notes Colleen Kelley, an infectious disease specialist at Emory University School of Medicine in Atlanta, Georgia, and a site investigator for the Novavax trial, which is using the randomization strategy.
Another workaround is a trial in which no placebos are involved, and a vaccine is instead compared with an already authorized one. The French vaccine manufacturer Sanofi Pasteur and its British partner GlaxoSmithKline are working on a protein-based vaccine similar to Novavax’s, and they are now planning studies with such a design. But, according to unpublished calculations from the biostatistics group of the US government’s Operation Warp Speed vaccine programme, proving that an experimental vaccine is not substantially inferior to one that is 95% effective would generally require trials that are longer and larger than placebo-controlled studies.
“They would need to be so big that they’re not likely to be practical,” says Peter Smith, an epidemiologist at the London School of Hygiene and Tropical Medicine.
Proxy of protection
Another option, therefore, is to measure a vaccine’s effectiveness by using immune markers that a person develops in their blood after inoculation. These are telltale signs — a certain level of antibody, say — of whether the immune system is primed to wipe out incoming coronaviruses.
New vaccines for influenza, rabies and many other infectious diseases are already evaluated1 using these ‘correlates of protection’, and this removes the need for placebos. The problem for COVID-19 vaccine developers is that, unlike the case with those diseases, it is not yet clear what kind of immune response is a reliable indicator of vaccine-induced protection against the coronavirus.
Trials so far suggest that levels of an antibody called immunoglobulin G (IgG) could serve as that proxy indicator, but the evidence is only circumstantial, says Dan Barouch, a virologist at Beth Israel Deaconess Medical Center in Boston, Massachusetts. To confirm IgG as a correlate of protection, scientists need to study people who have received a COVID-19 vaccine but then get sick anyway — developing what are called breakthrough infections. If the level of IgG in those people’s blood falls below a threshold found in people for whom the vaccines worked, that could help scientists determine the amount of the antibody needed for any new vaccine to be judged effective.
Ill disposed
Every large vaccine trial now in progress is testing people’s blood in search of potential correlates of protection. Yet, with so few breakthrough cases in many of the first-generation trials — just 11 in the study from US biotech company Moderna2 and 8 in the study from drug giant Pfizer and German biotech firm BioNTech3 during primary analysis — researchers will probably have to pool data across studies and vaccine platforms to get answers.
A further complication is that most large vaccine trials are designed to test whether participants develop symptoms of COVID-19, rather than whether they’ve been infected by the coronavirus. But if vaccines are to stop the spread of the virus, developers will require an immune correlate indicating that a person is protected from infection, not just from symptoms. This is something that is actively being investigated, but only through intermittent measurements that, according to Holly Janes, a biostatistician at the Fred Hutchinson Cancer Research Center in Seattle, Washington, “might miss infections”.
Some researchers argue in favour of deliberately exposing vaccinated individuals to coronavirus in ‘human challenge’ trials and then carefully tracking rates of infection and their accompanying biomarkers. The approach still typically involves placebos, but it requires far fewer volunteers than field trials and yields results far more quickly. Challenge trials remain controversial — unethical even, some say. But purely on a scientific level, everyone agrees that they are the best way to get accurate immune correlates, notes Nir Eyal, a bioethicist at Rutgers University in New Brunswick, New Jersey, and a proponent of the strategy.
Regulatory agencies are now working with scientists and vaccine companies to determine the best development path for next-gen vaccines. “We’re in this funny zone now,” says Rob Coleman, cofounder and chief executive of Codagenix, a company in Farmingdale, New York, that is due to begin human testing next week with a COVID-19 vaccine candidate that contains a weakened form of the coronavirus. “There’s no clear guidance.”

