Donald Trump’s COVID-19 treatment is similar to the average American hospitalized with coronavirus. Only faster.
USA Today – October 7, 2020
Roger Shapiro, MD (Infectious Diseases, BIDMC) discusses the use of steroids in COVID-19 treatment.
President Donald Trump has had the best possible care for his COVID-19, his doctors have repeatedly assured America.
“He’s on a routine regimen of COVID therapy,” his physician Sean Conley said Monday.
It’s true that while he’s had outstanding care, Trump’s therapies have been similar to those available to most other hospitalized COVID-19 patients – with two major exceptions.
The president received a dose of an experimental drug only tried so far in about 2,000 people as part of a research trial, and he’s gotten everything far sooner than would a typical COVID-19 patient.
Here’s a breakdown of the president’s treatment and how it differed from that available to most Americans:
Earlier on everything
Even his diagnosis came much faster.
Average Americans wait a few days after they start feeling sick to get COVID-19 tests – if they can even get one – and days longer for results. But Trump was tested and his positive diagnosis confirmed on Thursday, within 24 hours of first feeling fatigued on a flight back to Washington, D.C., from a rally in Minnesota.
He rapidly received the one nonstandard drug, a combination of so-called monoclonal antibodies, and was hospitalized “out of an abundance of caution” on Friday afternoon, less than 48 hours after his diagnosis.
Most Americans can get a medical professional on the phone in that time frame, but would likely be told to stay at home, take a fever-reducer like Tylenol, and monitor their breathing.

Trump’s doctors have been somewhat cagey about the president’s early symptoms, other than to describe a high fever and low energy.
His blood oxygen saturation level – a measure of the amount of oxygen in the blood – may have briefly fallen as low as the 80s twice on Friday and Saturday, Conley hinted. But it bounced back after he received oxygen, Conley said, reassuringly, and measured a normal 95% to 97% on Tuesday.
Most people wouldn’t know if they had oxygen level drops like that, unless they have a pulse oximeter at home, but usually would be admitted to the hospital if their oxygen levels dropped below about 94%, especially if they were having trouble breathing.
Monoclonal antibodies
Before Trump headed to the hospital, less than 48 hours after his first symptoms, he had already received a dose of a monoclonal antibody made by Regeneron, a New York biotech company.
The drug, REGN-COV2, is intended to mimic the natural process of the immune system, providing it with molecules called antibodies the body normally manufactures to fight off specific diseases. It is currently being tested in people at various stages of the disease, including patients who have been diagnosed and are symptomatic but not hospitalized, as was Trump.
REGN-COV2 includes two antibodies. One comes from a person who recovered from COVID-19, the other is from a mouse engineered to have a human immune system. Both target a protein on the surface of the coronavirus that causes COVID-19.
This drug and similar monoclonal antibodies, including one made by Eli Lilly and Co., of Indianapolis, remain under development and are not yet approved for use in the U.S. or elsewhere. Trump was able to get it under a “compassionate use” exemption, which the company said it has granted to fewer than 10 people so far, after requests from their doctors and approval by the U.S. Food and Drug Administration, a process that typically takes days to weeks.
Otherwise, about 2,000 people have taken REGN-COV2 as part of clinical trials designed to ensure that it is safe and effective.
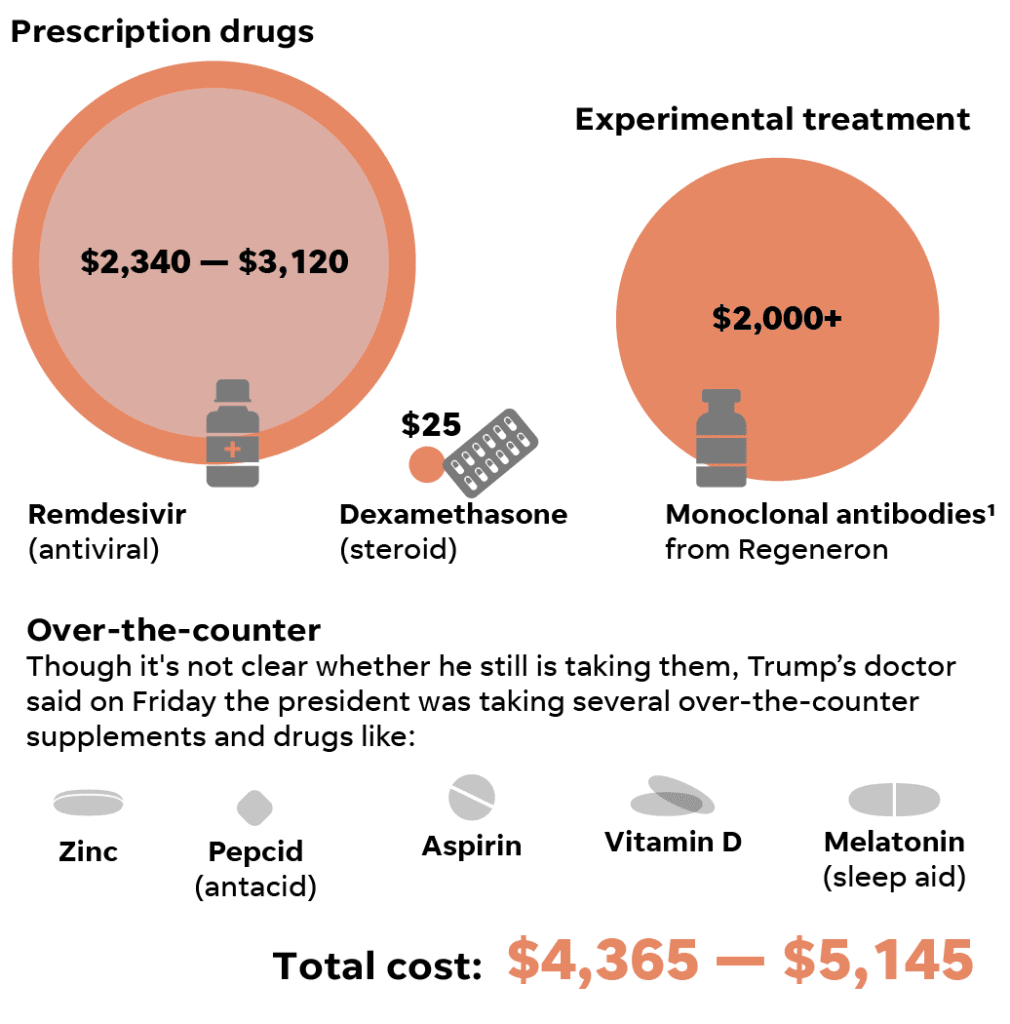
Trump and the others wouldn’t have been charged for the drug because it is not available for sale. Trial participants receive a stipend to compensate them for their time.
Prices aren’t set for drugs until they’re approved by the FDA.
But monoclonal antibodies are usually extremely expensive. Cancer-related monoclonal antibodies often cost nearly $100,000 a year, according to one 2018 study. For COVID-19, monoclonal antibodies are expected to be orders of magnitude cheaper, likely on the order of a few thousand dollars for a single-dose regimen.
Supply will likely remain tight even after approval because such biologic drugs are complex and time-consuming to manufacture. The company estimates between 70,000 and 300,000 treatment doses will be available under an agreement with the U.S. government, which would provide the treatment for free to COVID-19 patients.
Doctors have long put a lot of faith in monoclonal antibodies.
Dr. Rajesh Gandhi, an infectious disease specialist at Massachusetts General Hospital and Harvard Medical School in Boston, described monoclonal antibodies as “one of the more promising medications.”
“It’s really interesting and potentially important,” he said because it has the potential, when given early as it was to Trump, to prevent infections from becoming serious.
Remdesivir
Soon after arriving at the hospital Friday evening, Trump was given the first of five doses of remdesivir, an antiviral. The drug, made by Gilead Sciences of California, was developed to treat Ebola but has been repurposed to use against COVID-19.
It has been shown effective in patients who are hospitalized with breathing problems, but not sick enough to require ventilation. In that group, research has shown remdesivir can reduce the time a patient has to stay in the hospital, although three different trials of the drug have shown three different results.
Theoretically, because the drug is an antiviral, it should be more effective the earlier it is given in the course of disease, when the virus is just taking hold. But remdesivir has to be delivered intravenously at the moment (it may eventually be available in an inhaled form or as a pill), so most of the people who have taken it, and nearly 90% of the patients studied in the trial were defined as having “severe disease,” so were likely far along in their infection.
Trump got it on Day 2.
Sold under the name Veklury, remdesivir has been in shortage in the United States for most of the summer, though that seems to be easing. “Gilead is now meeting real-time demand for Veklury in the United States and anticipates meeting global demand for Veklury in October, even in the event of potential future surges of COVID-19,” according to a company news release from last week.
Private insurance companies will pay $520 per vial or $3,120 for a five-day course of treatment, while the company will charge the U.S. government $390 per vial, or $2,340 per patient.
“At the level we have priced remdesivir and with government programs in place, along with additional Gilead assistance as needed, we believe all patients will have access,” the company’s chairman and CEO, Daniel O’Day, said in June in announcing the pricing.
Most Americans hospitalized with COVID-19 now get remdesivir.
It’s a good drug, but not a game changer, said John Wherry, director of the Penn Institute of Immunology, at the University of Pennsylvania Perelman School of Medicine. “Remdesivr does work to some extent, especially when treating early in the disease,” he said.
Dexamethasone
Late Saturday, three days after his first symptoms and two days after his diagnosis, Trump was given the steroid dexamethasone.
This drug, though cheap and accessible, is usually reserved for people with very low oxygen saturation levels or those on ventilators. Because of the lack of data on Trump’s oxygen levels, it’s unclear whether he met those criteria.
Steroids do not provide a benefit when given to people in earlier stages of COVID-19, studies have shown, and can even be dangerous because they tamp down the immune system’s ability to fight off the virus.
Dexamethasone and other steroids are off-patent and inexpensive – just $25 for a 4 mg dose, according to the website drugs.com, while 10 mg costs about $35. They are widely used in the U.S. and around the world. The dose tested in patients with COVID-19 was 6 mg, though it’s unclear how much Trump is taking.
Learning about their effectiveness, especially for especially ill patients, has been a boon to COVID-19 treatment everywhere, said Dr. Roger Shapiro, an infectious disease specialist at the Harvard T. H. Chan School of Public Health, Harvard Medical School and Beth Israel Deaconess Medical Center, all in Boston.
“Steroids were a real success story for people with severe disease,” he said. “The reduction in mortality was really striking.”
But Shapiro said he was surprised that Trump received steroids so soon after his diagnosis. “Most everyone expects that steroids do the most good in the late stages,” he said. “That’s why a lot of people were scratching their heads about Trump getting steroids so early in the illness.”
Other important advances
The president has now exhausted the available care options.
No other treatments have been authorized for COVID-19, though a large number are in clinical trials, including some that help reduce immune overreactions that are common in COVID-19 and others that aim to prevent potentially lethal blood clots.
If Trump’s condition doesn’t continue to improve, his care will likely be the same as everyone else’s: mostly what’s called supportive care, like oxygen and fluids.
Most very ill patients, particularly at major teaching hospitals, are encouraged to participate in clinical trials – potentially helping themselves and others, said Dr. Gabriela Andujar Vazquez, an infectious disease specialist at Tufts Medical Center and Tufts University Medical School in Boston.
“You come to Tufts, you would be offered every single thing that’s has been put out there that seems to be safe,” she said.
Although there are no numbers on how many deaths are now prevented by improved medical care, doctors treating COVID-19 patients agree care has improved since the winter and spring when COVID-19 was brand new and some hospitals were totally overwhelmed with patients.
“Across the country, we’ve gotten better at treating people,” said Dr. Mark Rupp, chief of the division of infectious diseases at Nebraska Medical Center in Omaha. “I think we’ve gotten better at what the virus looks like and what we can expect and the supportive care that goes along with it.”
Doctors also have learned about treatments that aren’t helpful, like a combination of HIV drugs tested early on and shown to be ineffective.
And they’re no longer throwing the kitchen sink at their patients, Rupp said.
“What we call ‘compassionate release of medications’ is really not always compassionate,” he said, warning that doctors who provide such overtreatment can endanger people in their care. “Your heart is in the right place, you’re wanting to do good, but you’ve lost sight of the science and the data and being true to that.”
The next step for researchers, Gandhi said, will be studying how drugs like remdesivir and dexamethasone work together and the best timing for both, as well as adding those drugs to others to see if outcomes improve.
On Monday, Conley, Trump’s physician, said the president was healthy enough to go home, an assurance that concerned many health professionals who saw him later on a White House balcony, apparently struggling to breathe.
“Though he may not be entirely out of the woods yet, the team and I agree that all our evaluations, and most importantly, his clinical status, support the president’s safe return home,” Conley said.
Of course, the president’s home is not like any other. “He’ll be surrounded by world-class medical care, 24/7,” Conley concluded.

