Dan Barouch, MD, PhD (Center for Virology and Vaccine Research, BIDMC) discusses his team’s work on developing a potential vaccine to prevent COVID-19.
Boston Magazine – June 4, 2020
The Untold Story of Moderna’s Race for a COVID-19 Vaccine
During the small hours of a cold January morning, Stéphane Bancel slipped into the kitchen while his wife and children were still in bed. He was technically on vacation at his home in the south of France, but even on his days off, Bancel didn’t sleep much. He put on the kettle and fired up his iPad. From his kitchen window, he had a full view of the sea. The sun had not yet emerged from behind the horizon, but already color was leaking across the sky and rippling on the surface of the water, hinting at its imminent and fiery arrival.
Bancel fixed himself a cup of black tea and took a seat at the counter to kick off his early-morning ritual with the Wall Street Journal. At 47 years old, his dark hair was thinning but his French accent was as thick as it had been nearly a decade earlier, when he mystified his colleagues by leaving a plum CEO job at a French multinational diagnostics company and moving to Boston to run Moderna—at the time a positively tiny Kendall Square biotech that was still operating in stealth mode. The Journal contained enough business news to keep Bancel occupied, but it was another headline that grabbed his attention—about the outbreak of a mysterious illness in China. He clicked on the story and scrolled down looking for details: 59 people sick with viral pneumonia. Up from 27 from just days before. The likely suspects had been ruled out. The culprit was a mystery.
Bancel, who’d spent much of his career at companies that focused on infectious disease, raised his eyebrows. He didn’t like the sound of it one bit, so he copied the story link and pasted it into an email directed at Barney Graham, deputy director of the Vaccine Research Center at the National Institutes of Health. “Can you make any sense of the viral outbreak going on in China?” Bancel pecked out on his tablet.
Moderna had worked with Graham and the NIH over the past few years on its quest to bring a whole new class of vaccines to market. Traditional vaccines are made from a weakened, dead, or small piece of a pathogen—a virus or bacteria—which prompts the body to fight off the invader and builds up immunity for when the real deal comes along. In contrast, Moderna’s vaccines contain an engineered strand of messenger RNA, which tells the body’s cells to produce a very specific part of a pathogen that will ignite an immune response. In other words, mRNA vaccines shift the site of vaccine production from the factory to the body itself.
Later that day, Bancel’s phone pinged with an email from Graham at the NIH. While he couldn’t yet identify it, Graham said, “If it’s a coronavirus, we know what to do and have proven mRNA is effective.”
The NIH’s confidence stemmed from Moderna’s early-stage human trial data from 2019. In fact, that data was so encouraging that Bancel was set to announce in a few days’ time that the company would be doubling down on its vaccine-development program in 2020, with hopes of getting the world’s first mRNA vaccine—and what would be Moderna’s first licensed product—onto the market in the next few years.
The platform’s potential seemed limitless. If ultimately proven successful in large-scale human trials, mRNA vaccines would herald nothing short of a revolution akin to the advent of the steam engine when it comes to preventing infectious disease. For one thing, because mRNA vaccines don’t contain a pathogen, they are thought to be safer than traditional vaccines. They are also relatively easy to scale and produce—you can design any mRNA vaccine in the world on a computer and manufacture it in a single facility regardless of what disease it is for. Most important, using mRNA is a much faster way to make vaccines, which can normally take up to a decade to develop and test.

Bancel forwarded Graham’s email to Hamilton Bennett, an energetic and idealistic 35-year-old who’d spent the previous three years as program leader on Moderna’s vaccine portfolio. Three days later, on January 10, Bennett was on a previously scheduled call with Graham when the subject of China’s low-level viral outbreak came up. It was 7 p.m. on a Friday, and the streets below in Kendall Square had already emptied out for the weekend when Graham asked Bennett if Moderna would be interested in using the new virus to test the company’s accelerated vaccine-making capabilities. That way, Graham mused, if ever there came a day when a new virus emerged that threatened global public health, Moderna and the NIH could know how long it would take them to respond.
Bennett was no stranger to working at breakneck speed. She had managed Moderna’s Zika program that produced a vaccine in a mere 10 months, a company record. No one, not even Bennett, knew just how fast the company could deliver this vaccine, but she wanted to find out. She agreed that as soon as the new virus’s sequence was publicly posted online, sometime in the coming days, her team would begin a dry run. Because mRNA vaccines are made on a computer and not from the viruses themselves, that was all they needed to begin.
That weekend the Chinese government posted the virus’s sequence. Early Monday morning, Moderna’s scientists went to work on a COVID-19 vaccine. It was January 13. The clock was officially ticking.

Several days later, on a moonlit Friday night, Bancel wheeled his suitcase into Terminal D at Logan Airport and checked into a red-eye bound for Zurich. He was heading toward the snow-cloaked Alps for the World Economic Forum at the Davos ski resort, where corporate leaders meet each year in a shared desire to save the world through capitalism. But on the sidelines, far from the spotlight of the main stage, much of the chatter was about the virus spreading through China. Meanwhile, as Chinese officials assured the world that the outbreak wouldn’t be as bad as SARS, a 2003 coronavirus that sickened 8,100 people and killed 774, Bancel was hearing a far darker version of the future.
As he spent time with two prominent infectious-disease experts from Europe, they were receiving a constant stream of information from their contacts on the ground in China, including Wuhan, the epicenter of the outbreak. The data showed sky-high infection rates and mounting evidence that human-to-human transmission was well under way. Much of it wasn’t even public and yet here they were, turning their phone screens toward Bancel for him to see. That evening, when Bancel returned to his hotel in the famous but aged ski resort, his mind was reeling from what he had heard and seen. His room was cold and tiny, consisting of little more than a single bed that evoked his childhood bedroom in France, a tiny desk, and an extra heater he had requested from reception. As he huddled next to the heater, he searched Google for international flights out of Wuhan. What he saw sent shockwaves down to the tips of his fingers: an endless list of flights to nearly every corner of the world. The cat is out of the bag, he thought. This isn’t going to be SARS. It’s going to be the 1918 flu pandemic.
Bancel knew he needed to reach out to Moderna cofounder Noubar Afeyan, the serial biotech entrepreneur who runs Flagship Pioneering in Cambridge and chairs Moderna’s board of directors. Bancel texted him: We need to talk. At the time, Afeyan was sitting at a marble-topped table at Pammy’s in Cambridge celebrating his daughter’s birthday. Normally, he would never take a call at a moment like that, but Bancel usually only communicated with him through texts—unless, that is, the matter was urgent. Afeyan apologized to his daughter, quickly rose from the table, and without putting on his coat walked out of the restaurant and onto Mass. Ave. to take the call. Bancel told him what he’d learned about the virus and suggested that Moderna begin to build the vaccine—for real. They agreed to convene the executive committee to discuss it straightaway.
The next day, Bancel dialed into the meeting with the executive leaders, who were crowded around a conference table at Moderna’s headquarters with Bennett and the members of her team that had been tirelessly working on what up until that point essentially amounted to a demonstration project. At first, some committee members urged caution. After all, this would distract from their carefully plotted course for 2020, not to mention disrupt a young company still working to bring its first drug to market. Moderna scientists had never tried to make a vaccine this quickly before, and there was risk of straining the platform and the team beyond their breaking points. In addition, what if a vaccine wasn’t needed? Pharma companies had gotten left holding the bag during other novel viral epidemics, investing heavily to develop vaccines only to watch as the disease fizzled out on its own. On the flip side, what if it really was needed? If so, Moderna’s race for the vaccine would play out under a million-watt global spotlight. The company would either celebrate one hell of public market debut, or fail while the whole world watched.
Bennett argued to go for the real vaccine. Moderna was perfectly poised to respond and had an obligation to do so, she said. To Bancel, meanwhile, the sheer act of debating whether to move forward struck him as absurd in light of what he was learning at Davos. In his world, a global pandemic was about to descend like a biblical plague, and whatever distractions the vaccine caused internally at Moderna were irrelevant. Ultimately, the committee agreed to go for it.
Back in the lab, Bennett’s team began transforming the test project into a full-blown vaccine program, while Bancel continued to do what he does best—build partnerships, generate excitement, and secure funding. In Davos, he hammered out a collaboration agreement with the Coalition for Epidemic Preparedness Innovations (CEPI), a foundation that finances infectious-disease research, to produce Phase 1 of the vaccine. On January 23, Moderna and CEPI went public with the news—but Bancel’s attention was focused on the information coming out of Wuhan that day. Chinese officials had closed the airport, bus terminals, and train stations, effectively shuttering a city of 11 million. Bancel shook his head. He had been through epidemics before, but he had never seen anything like this.
Moderna may have been one of the first biotechs out of the gate in hot pursuit of a COVID-19 vaccine, but it soon had company. The day CEPI announced it was funding Moderna’s Phase 1 trial, it also announced it would fund a University of Queensland lab and Pennsylvania-based Inovio Pharmaceuticals. Like Moderna, both of these groups utilize novel platforms that can quickly make vaccines without using the virus itself. In the early days of the race, Inovio CEO Joseph Kim seemed to relish the competition. “This is a great opportunity to go mano a mano with Moderna,” he told a reporter from Science magazine.
When it came to Big Pharma, there was less enthusiasm to jump in. On an industry conference call about emerging infectious diseases on January 29, Bennett listened in as representatives from major pharmaceutical companies hedged and dodged. It was too soon, they said. They wanted to see how it would play out. One executive mentioned that several biotechs were chasing the vaccine. “We will see what they can do,” he said.
“Okay,” Bennett said under her breath, her microphone on mute. “We will show you what we can do.”
Across the Charles in Boston, there was one scientist who thought Big Pharma should get on board—and he had a feeling he could make it happen. Dan Barouch, who heads the Center for Virology and Vaccine Research at Beth Israel Deaconess Medical Center, is something of a prodigy, having run his own research lab since the age of 29. Almost twenty years later, he’s still baby faced and his hair is still jet black, though he’s no longer always the youngest guy in the room.
Barouch is no stranger to chasing vaccines. He has spent most of his career seeking one for HIV and has worked on one for Ebola, too. But it was his work during the 2016 Zika outbreak that rocketed him to broader fame after he produced an investigational vaccine in a then-unheard-of 180 days. Barouch has been pursuing using another innovative platform called adenoviral vector vaccines, which use a common cold virus as a vector to shuttle a piece of DNA into a cell. Once inside the cell, that piece of DNA codes for a specific piece of the pathogen that sparks the desired immune response. The United States has yet to approve a vaccine made this way, though large-scale human trials are under way.
Barouch’s lab had already designed its own COVID-19 virus vaccine as an academic exercise soon after the sequence became available. By the weekend of January 25, just days after Moderna decided to go full steam ahead, he was convinced the world was going to need a vaccine for this virus. What’s more, Barouch was confident his platform was the best one to deliver it. And he knew just whom to call.
Barouch had worked in the past with Janssen, the pharmaceutical arm of Johnson & Johnson. Still, that was no guarantee he’d get a response on a Saturday from a pharma exec. As he followed the news out of China, though, he felt a rising sense of urgency. Sitting in his lab, Barouch fired off an email to Johan Van Hoof, Janssen’s global head of vaccines. “We are going to need a vaccine for this,” he wrote. “We need to talk.”
Within an hour, Barouch had his reply. “Can I call you right now?”
They spent several hours on the phone that day, and by the time Monday rolled around they had agreed to join forces once again, capitalizing on Barouch’s ability to design a vaccine and carry out preclinical work in the lab, and Janssen’s manufacturing capabilities and sheer muscle as a major pharma company. As they ironed out the details of their partnership, public health officials delivered sobering news to the American public: Illinois had logged the United States’ first recorded case of community transmission. Up until then, all of the U.S. COVID-19 cases had been imported. This marked the first time the virus had spread on our home turf.
Usually partnerships between academic labs and pharma take months to negotiate. Six days after beginning their conversation, on January 31, Barouch’s lab and Janssen had an agreement signed, sealed, and delivered. Big Pharma had officially joined the race.
At least initially, Bennett didn’t know how long it would take Moderna to make its first COVID-19 vaccine. At 8:30 a.m. one morning during the early days of the project, a member of her team delivered the estimated timeline: 120 days. “That’s insane,” Bennett replied, aware that the previous record—which she had set—was 10 months. “Are you sure?”
“Yeah,” her colleague said. “We are comfortable with that.”
As fast as they were working in Moderna’s state-of-the-art Norwood plant, everyone wanted to move faster. During a meeting one morning, an engineer suggested that “the virus doesn’t take weekends, so we can’t either.” Weekends were officially canceled. The virus doesn’t knock off at 6 p.m. for that matter, so team members agreed to organize themselves in shifts so someone would always be driving production forward, all day, every day.
Soon, other decisions were made in the name of speed. Usually vaccines are made sequentially: You wait for lab tests to reveal that your vaccine candidate is working before you manufacture an animal-grade vaccine. Then you wait for the animal data before you manufacture a vaccine for human trials. Since time was of the essence, Moderna decided to do all of the steps simultaneously. It was a gamble. The team would be well into the next step before it even knew if results from the previous step indicated they were on the right path. If they weren’t, their time, effort, and resources would be wasted, and they’d have to backtrack and start over. But if the risk paid off, the reward would be substantial: It could shave weeks, even months, off the timeline.
The plan worked. Bennett had just entered the estimated 120-day timetable into her project-management software and advised the NIH of the schedule when manufacturing came back and said they could do it even faster. Bennett went back into the software and updated everyone’s timeline. This happened so many times that she eventually ditched the software altogether. From that point on, every time she received a new, accelerated timeline, she’d simply stand up, wipe the old one off the giant whiteboard that extended the length of her desk, and mark the new one down. Before she knew it, they had halved the time to 60 days.
As the days and nights ground on, the intensity and relentless rhythm of the work started to wear on Bennett. Seven days a week, she stayed late at the office before driving home to Malden, where her husband had long ago gone to sleep. On February 6, Bennett had just left work when her car broke down. It had left her stranded a few times since the project began (exactly how often was another metric that the consummate planner was tracking on her whiteboard). That night, she ditched her vehicle near the office, called an Uber, and managed to hold it together the whole ride home. As soon as she walked into her house, though, she dropped her bag on the floor and fell into a puddle of tears. The next day, she was back in the office at 7 a.m.
Everyone at Moderna was on tenterhooks waiting for manufacturing to finish making the vaccine. Each day, as the coronavirus advanced around the globe, the quest for a vaccine took on an even higher level of importance and became a greater sense of pride for workers at the company—even for some far away from the manufacturing room floor.
On February 7, the chief of technical operations and quality, industry veteran Juan Andres, went to the cafeteria at the Norwood plant for lunch. When he reached the head of the queue and placed his order, the line cook asked a question as he heaped a scoop of chicken salad onto Andres’s sandwich.
“How’s the vaccine going?” he said. “When will it be done?”
Andres—who was expecting something more along the lines of, Do you want lettuce and tomato on that?—was taken aback. That’s when he realized: Everyone was following the project’s progress.
“It’s going well,” Andres told him. “It should be done today.”
He did not disappoint. Later that afternoon, the batch was finished. It had taken only 25 days. Now came the agonizing waiting game. It would need to undergo a series of experiments, the longest of which was the requisite sterility test for any product injected into humans. It took 14 days, and there was no way to shave any time off that one.
Bancel proudly refers to himself as a paranoid optimist. “I believe in science and the possibility of great teams coming together to do things that were thought to be impossible,” he says. “But I also know that shit happens.” So as upbeat as he felt about Moderna’s efforts to build a vaccine, he was gripped with fear every day of the manufacturing process that something would go wrong. Nothing, however, produced a more acute case of agita for Bancel than the topic of how to ship the vaccine to the NIH when it was finished.
Bancel had been burned by shipping before. Not so long ago, one of Moderna’s products was left outside of cold storage once it was no longer in the company’s hands, and the entire load had to be tossed in the trash. Now, his company had embarked on a timed trial that was doubling as the government’s vaccine response to a global pandemic, and Bancel was not going to let something as trivial as a shipping error derail them.
Over several days, Bancel gathered with colleagues to plot out a foolproof shipping strategy. As he brainstormed options with Andres—someone Bancel affectionately calls as paranoid as he is—their solutions quickly spun out of hand. At one point, they started having a fantastical discussion about using some combination of high-end cold-chain logistics combined with Brinks-style security. At another, they considered putting a Moderna employee on the truck to personally chaperone the vials to the NIH in Maryland. Someone less prone to paranoia finally talked them off the ledge, and Bancel settled on planting a GPS chip in the package so Moderna could track its golden goose the entire way.
Aside from shipping, the sterility test was Bancel’s biggest worry. “There is no gray area; it is either 100 percent sterile or you are done,” he says. “You cannot fix an unsterile product—you just put it in the garbage and start again from scratch.”
On the 14th and final day of the sterility test, the quality control team told Bancel they would not have any news until later that evening. Bancel made sure the ringer on his phone was on before he left the office and made his way across the Longfellow Bridge to his home on Beacon Hill. During dinner, the dings of incoming text messages punctuated his conversation. Each time, he stole a glance at his phone to see if it was the only message he cared about: the results. Nothing. Later that evening, he was standing in his kitchen when his phone dinged again. He lunged for it. The message came from the director of quality control. The batch was sterile—it had passed the test. Bancel felt a 500-pound weight lift off his shoulders.
Back at the plant the next day, the team prepared the vials for shipping. When they were ready to go, the GPS chips tucked safely inside, there was one last thing to do: The team crowded around the boxes, with big smiles and thumbs up, and snapped a selfie. It had been 42 days since they designed the vaccine. They had already made history.
Under the lights on the loading dock, they secured the precious cargo in the truck and closed the door. The vehicle pulled out of the plant, its taillights glowing red in the dark as it headed south to Maryland.
Jennifer Haller and her husband were driving to a hockey game south of Seattle on February 29 when she heard on the radio that the United States had suffered its first COVID-19 death. The victim was a resident of a long-term-care facility in Kirkland, a suburb of Seattle that borders Kenmore, the town where Haller’s mom and stepfather live. Health officials warned that it was just the tip of the iceberg. But it wasn’t until a few days later, as the deaths in Kirkland mounted, that Haller began to really worry about her 85-year-old stepdad. He had two vulnerabilities: asthma and a particular fondness for Mexican fast food. A retiree, his big daily event was climbing into his car and making a run south of the border—of Kenmore, that is—to neighboring Kirkland’s Taco Bell.
Haller picked up the phone and called her mother. “Just about the last place he needs to be is in a fast-food restaurant in Kirkland,” she told her mom. “He needs to just stop until we know what’s going on.” Haller’s mother agreed, but it did little to alleviate Haller’s sense of helplessness. She felt as though there was nothing she could do.
News of the first U.S. death and the spread of the coronavirus in Seattle were also on Bancel’s mind, but for slightly different reasons. Any day now, Moderna was expecting the green light from the FDA, allowing the NIH to move ahead with a Phase 1 trial for the company’s new vaccine. The site that the NIH had chosen for the trial—the only site it had selected—was in Seattle. Bancel was nervous. He knew that if Seattle became too much of a hot spot, it could upend the trial in many ways. He texted with Afeyan and emailed with Bennett, expressing his fears that volunteers might not want to go to the hospital to participate if it got bad enough in Seattle. Ultimately, he decided he’d leave it to Anthony Fauci’s team at the NIH to determine what was best—they were the pros, after all.
Two days later, on March 2, Bancel flew to the nation’s capital and got into a car bound for the White House. As he made his way past security and into the West Wing, Bancel, who still thought of himself as a middle-class kid from the south of France despite having amassed a fortune after Moderna’s record-breaking 2018 IPO, felt humbled by the invitation. Bancel would be meeting with President Donald Trump alongside the CEOs of the world’s biggest and most powerful pharmaceutical giants, including Janssen, GlaxoSmithKline, and Sanofi—companies with dozens, in some cases hundreds, of drugs on the market and market caps north of $100 billion. It was as if overnight, Moderna had been bumped up to the adults’ table and it was Christmas dinner.
From the start, Bancel felt the pressure of the spotlight. Before the meeting officially began, one of the president’s aides introduced Trump to Bancel, explaining that Moderna had the first vaccine ready for trial. Trump looked him in the eye and asked, “Can I count on you? Can you get it done?”
“Yes, sir,” Bancel replied.
During the meeting, Bancel explained to Trump that he was waiting for FDA approval for the study and that Moderna would soon be testing the vaccine in humans. That very day, after Bancel left the White House to head back to Boston, the FDA sent word that they were approving the trial. It was showtime.
At the Kaiser Permanente Washington Health Research Institute in Seattle, study recruiters leapt into action. Kaiser needed to find dozens of healthy people who would volunteer as guinea pigs for Moderna’s Phase 1 safety trial. A few days later, Haller was at her desk for the tech firm where she works in Seattle, taking a break and catching up with her Facebook feed, when she saw a post that Kaiser was searching for volunteers. As soon as she saw the ad, it hit her: Maybe there was something she could do after all. Haller quickly filled out the questionnaire and crossed her fingers.
“Shouldn’t we talk about this?” her husband asked when she told him that night that she’d signed up.
“Yeah,” Haller said. “Sure. But I’m doing it anyway.”
Her husband already sensed that no one was going to stop her. Days later, once she found out she’d been selected, several friends tried to dissuade her. “This sounds risky,” one of them told her. “You know you don’t have to do this.”
Haller wasn’t fazed. She felt fortunate to have a job she could do from home, and because her hours were flexible, she knew she could make it to the dozen or so required appointments. In other words, she was in a position to help, and felt compelled to do so. She might not be on the frontline, but she at least wanted to be on the battlefield.
As Haller waited for the study to start, the deadly virus continued to spread. On March 11, Italy closed for business. The same day, the sober-faced director-general of the World Health Organization faced the media and uttered the words no one wanted to hear: COVID-19 was officially a global pandemic. The news only strengthened Haller’s resolve to participate in Moderna’s trials. She signed a 45-page informed consent document listing the medical risks without thinking twice. This vaccine process needs to happen soon, she told herself. Let’s get this started.
On March 16, Haller woke up early, put on a tank top, threw a sweatshirt over it, and downed a scrambled egg that her husband had prepared. Upstairs, her kids were still asleep when she slid into her car and drove downtown. On the radio, the news was wall-to-wall coronavirus.
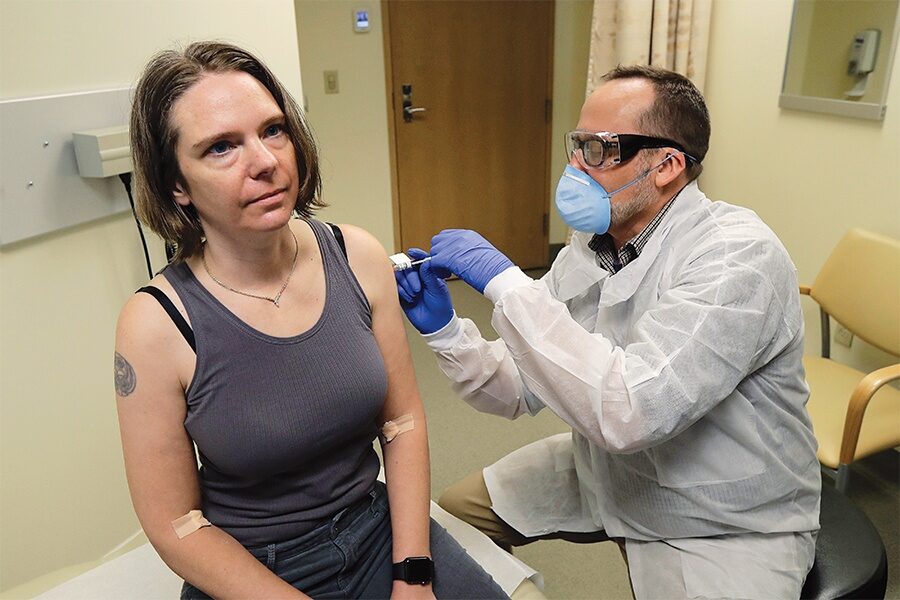
When Haller arrived at the Kaiser building, she pulled into the garage and walked into the clinic, where she learned she would be the very first person in the world to receive the injection. An Associated Press news crew was there to film the historic event, and it hit her how important this moment was, for everyone. She needed to sign off on some final risks—including the fact that the vaccine had not gone through a complete round of animal testing—before proceeding. In a decision that has been controversial in some corners of the scientific community, the FDA allowed Moderna, based on prior animal data, to try its vaccine on humans without complete animal trials. Haller wasn’t worried. She signed away.
Next, a pharmacist readied the injection. Haller felt a small prick in her left arm, followed by the sensation of the syringe’s cool liquid contents slipping into her biceps, and the rising satisfaction of knowing that she had finally found a way to do something.
On March 23, Governor Charlie Baker stood before a crush of reporters and made it official: All nonessential businesses were ordered to close their doors and all residents of Massachusetts were instructed to stay at home and isolate from one another. Almost overnight, Boston’s streets became eerily quiet as life as we knew it drifted further into a new normal. People lost their jobs, diagnoses soared, and the body count rose.
Throughout Boston and beyond, however, there had never been a more active moment in the world of vaccine research. The day after Haller was dosed, CanSinoBio in China announced it had received approval for a Phase 1 trial. On March 30, Johnson & Johnson announced it was investing a billion dollars for Janssen to create a billion doses of Barouch’s vaccine if it proved successful. His vaccine should make it to a Phase 1 trial by September, but Barouch says that his platform is merely a slow starter and will make up time in the homestretch, meaning it could be first to market. On April 6, Inovio, the Pennsylvania company, announced it was approved to start its Phase 1 trial. Soon after, the U.S. government announced it was funding Moderna with nearly half a billion dollars. The news sent Moderna’s stock price so high that Bancel became a billionaire overnight.
In late April, Moderna was still at the front of the pack, planning on Phase 2 trials this summer and then Phase 3 trials in the fall. Then, out of nowhere, an Oxford University lab with an experimental vaccine received government permission to jump ahead to Phase 2 based on its prior work, leapfrogging ahead of Moderna. By May, though, Moderna had pulled back into the lead when its Phase 2 trials were approved to start sooner than expected. On May 18, Moderna took a giant step forward and extended its margin, appearing on the front pages from New York to Hong Kong after announcing some of its study volunteers had tested positive for antibodies, with levels higher than people who actually had COVID-19. The promising news not only shot Moderna’s share price higher, but sent the entire stock market soaring by more than 900 points. Bancel says he might be ready to submit for FDA approval for the vaccine by year’s end.
At one point, there were more than 100 companies pursuing a vaccine, most of which will never make it into clinical trials. Of those that do, not all will make it to Phase 3, and no one knows which—if any—will ultimately prove effective and ostensibly save the world. For his part, Barouch says his vaccine and Moderna’s—both innovative new platforms trying to break into the big leagues—are among the leading candidates. Bancel, of course, hopes that Moderna’s vaccine works, but he doesn’t want to be alone in the winner’s circle. Even as Moderna announced a 10-year agreement with Swiss drugmaker Lonza to produce a billion doses a year, Bancel emphasized that no one company can supply the entire planet. “My hope for the world is that many of us get to the finish line, so we can stop this thing,” Bancel says. “The only way the world gets back to normal is with broad vaccination.”
In the meantime, at Moderna—and for all of us—the clock is still ticking.

