As millions of the Pfizer and Moderna COVID-19 vaccines are expected to be distributed before year’s end, Dan Barouch, MD, PhD (Center for Virology and Vaccine Research, BIDMC) says these two vaccines alone are not enough for rapid distribution in the U.S. and certainly not globally.
ABC News – December 17, 2020
More vaccines need authorization to inoculate all Americans
Millions of the Pfizer and Moderna COVID-19 vaccines are likely to be distributed before year’s end, but experts say more vaccines will need authorization if most Americans wish to be vaccinated in the months to come.
“These two vaccines alone are not enough,” said Dr. Dan Barouch, director of the Center for Virology and Vaccine Research at Beth Israel Deaconess Medical Center in Boston. “They’re not enough for a rapid distribution of vaccines in the United States and for sure they’re not enough globally.”
The United States has secured at least 300 million doses of Pfizer’s and Moderna’s via its Operation Warp Speed contracts. This could potentially inoculate 150 million Americans since these vaccines require two shots. The U.S. government is attempting to negotiate more doses from Pfizer and is reserving the right to buy more doses from Moderna.
At the moment, only tens of millions of doses are ready for distribution from these companies.
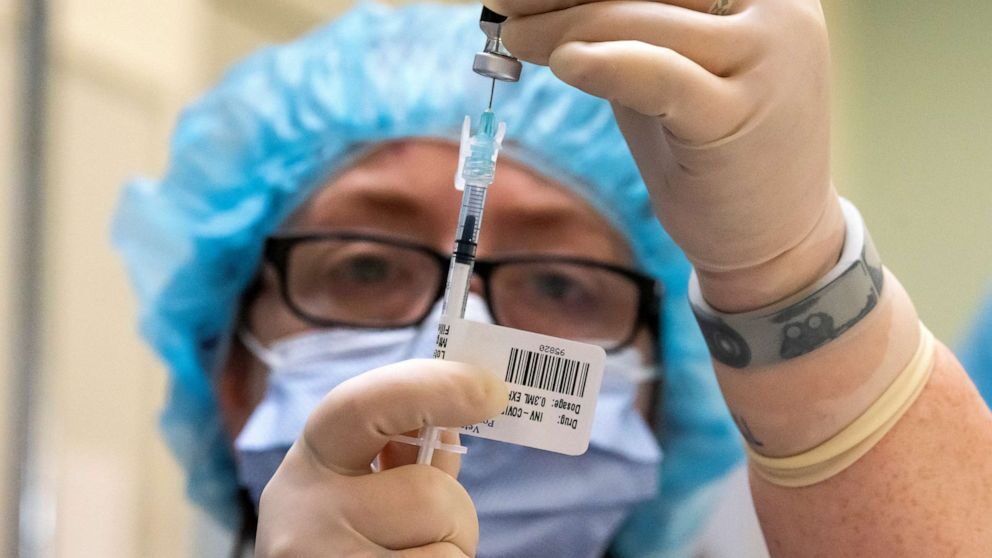
“The key here is to have multiple vaccines available where each manufacturer’s contributing their maximum amount to the pot, if you will,” said Soumi Saha, senior director of Premier, Inc. “We know that no single vaccine manufacturer can make six hundred and thirty million doses in order to vaccinate the entire US population.”
There are over 160 vaccines in development, and over 50 of them are undergoing human trials. While several of the top candidates are still on track — some even promising to be more efficient to distribute than Pfizer and Moderna — some top vaccine candidates have announced major setbacks.
And these vaccines are needed.
There are now over 307,000 confirmed COVID-related fatalities in the U.S. And in California, the state reported a record-high of 53,711 new cases Wednesday — a 3.3% increase from Tuesday. This surpassed the previous daily case record of 35,729, which was reported on Dec. 11. The Golden State recorded 293 additional deaths on Wednesday, up 1.4% from Tuesday.
The setbacks
AstraZeneca and Sanofi, two vaccine developers affiliated with Operation Warp Speed, hit speed bumps over the past few weeks.
A vaccine from Oxford University and AstraZeneca was between 60% and 90% effective in clinical trials depending on the dosage. Since the dosage required for optimal efficacy is still unclear, the company is considering launching new studies to figure out the optimal dose.
Although Sanofi tells ABC News it’s disappointed by the delay, its scientists will continue to work on its vaccine candidate.
“I think it’s important for these multiple [vaccines] to get into clinical trials,” Global Head of Vaccine R&D for Sanofi John Shiver told ABC News back in September, though at the time optimistic his vaccine would show greater immunity. “It’s not a competition against each other, it’s a competition against the virus — and the more successes there are vaccine wise, the better off we all are.”
While Sanofi’s vaccine timeline is now a bit murky, the U.K. expects its Medicines and Healthcare products Regulatory Agency (MHRA) to decide whether to authorize AstraZeneca’s vaccine shortly after the new year.
“It’s very normal for there to be delays,” Barouch said. “It’s very understandable that some programs have some setbacks along the way — in fact, it’s very rare not to have any setbacks.”
Through Operation Warp Speed, the U.S. government signed a deal to buy 300 million doses of AstraZeneca’s COVID vaccine and 100 million doses of Sanofi’s and GSK’s vaccine — the deals are only implemented if both are authorized for emergency use from the U.S. Food and Drug Administration.
Still on track
Two other companies, Novavax and Johnson and Johnson, are still on track with their timelines and are waiting for their late-stage trial results.
Johnson and Johnson’s candidate is of particular interest since its vaccine may only require one shot.
“The J&J vaccine has certain features that may make it very useful for a mass vaccination campaign,” Barouch, who helped develop the vaccine, said. “One of the features is that it’s highly immunogenic even with a single dose.”
Compared to the Pfizer and Moderna vaccines, patients will not have to schedule return trips to their pharmacies.
“When you think about the challenges of ensuring that someone comes in for two appointments … there can be some drop off in between that first and second shot,” said CVS Senior VP of Health Chris Cox.
If successful, Johnson and Johnson, along with Novavax, could produce another 200 million doses for the U.S. Barouch hopes to see Johnson and Johnson’s vaccine reviewed by the FDA in the early months of 2021.
“If there’s a positive signal — and I should emphasize — we don’t have any positive data yet, but if we are able to show safety and efficacy with the J&J vaccine by the end of January, then it could be potentially approved about a month later. So we’re talking end of February,” Barouch told ABC News.
Thursday, the FDA’s Vaccine and Related Biological Products Advisory Committee will review and provide recommendations on whether the benefits of the Moderna’s vaccine outweigh its risks for use in adults age 18 and older.
Two million more Pfizer doses are expected to be shipped through the U.S. next week, and if Moderna receives FDA authorization, officials expect just under 5.9 million of its vaccine will ship out along with them.
This report was featured in the Thursday, Dec. 17, 2020, episode of “Start Here,” ABC News’ daily news podcast.

