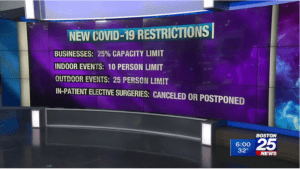
The contagious variant of the coronavirus spreading through Brittan has appeared in Colorado and California, but scientists are still unsure how much more easily the mutant spreads. Dan Barouch, MD, PhD (Center for Virology and Vaccine Research, BIDMC) spoke to the virus replication process and spread.
A more contagious version of the coronavirus may alter the course of the pandemic in the United States, researchers said.